ABOUT US
NEWS & EVENTS SERVICESCONTACT
- Dissolution Tester DS 8000 (Basic)
- Dissolution Tester DS 14000 (Basic)
- Dissolution Apparatus DS 8000 with Peristaltic Pump
- Tablet Dissolution Tester DS 14000 with Peristaltic Pump
- Dissolution Tester DS 8000+ with Syringe Pump
- Dissolution Tester DS 8000+ with Piston Pump
- Dissolution Tester DS 14000+ with Piston Pump
- Dissolution Tester DS 8000+ with Piston Pump & Automatic Filter Changer
- Dissolution Tester DS 8000 (Basic) SMART
- Dissolution Tester DS 14000 (Basic) SMART
- Dissolution Tester DS 8000 SMART with Piston Pump
- Dissolution Tester DS 14000 SMART with Piston Pump
- Dissolution Accessories
- Dissolution Tester Mechanical Calibration Toolkit
- Dissolution Tester DS 14000+ with Syringe Pump
- Cyclone Milll Twister TW1100
- Vibratory Disc Mill 1100
- Vibratory Disc Mill VDM 1200
- Vibratory Disc Mill VDM 1000 Series
- HIGH ENERGY BALL MILL
- Micro Ball Mill MM 1100
- Cryogenic Ball Mill CM 1100
- Planetary Ball Mill BM 1100+ (1 Grinding Station)
- Planetary Ball Mill BM 1400+ (4 Grinding Stations)
- Planetary Ball Mill BM 1200+
- Hammer Mill HM 1100
- Planetary Ball Mills BM 1500+ Series
- Planetary Ball Mill BM1150+ (Two Grinding Stations)
- Jaw Crusher JC1000
- Proximate Analyzer
- Pulverizer (Disc Mill)
- Automatic Pellet Press LP40T
- EDGE
- Microwave Digestion System Simultaneous (Mars 6)
- Microwave Synthesis Discover System
- Microwave Digestion Sequential
- Microwave Drying System
- Microwave Fat / Moisture Analyser
- Sprint Protein Analyser
- Microwave Peptide Synthesizer
- ORACLE - Rapid NMR Fat Analyzer
- Liberty PROTM
- Razor Rapid Peptide Cleavage SystemTM
- Phoenix BLACK
- Microwave Synthesizer Discover® 2.0
- PLF Series Chamber Furnaces ( PLF 140/5 - 160/30 )
- PLF Series Chamber Furnaces ( PLF 110/6 - 130/60 )
- MoS Series Chamber Furnaces
- ACF Series Atmosphere Controlled Furnaces
- ELV Series Elevating, Lift Bottom Furnaces
- HLF Series Heat Treatment Furnaces
- PTF Series Tube Furnaces
- PZF Series Multi-Zone Tube Furnaces
- STF Series Tube Furnaces
- PAF Series Ashing Furnace
- PURISPIN 18R
- Purispin 6
- PURISPIN 17R - Micro Centrifuge
- VARISPIN 15R - Multi Purpose Centrifuge
- VARISPIN 15 - Multi Purpose Centrifuge
- VARISPIN 12R - Multi Purpose Centrifuge
- VARISPIN 12 - Multi Purpose Centrifuge
- VARISPIN 4 - Multi Purpose Centrifuge
- VARISPIN 4A - Multi Purpose Centrifuge
- VELOSPIN 22R
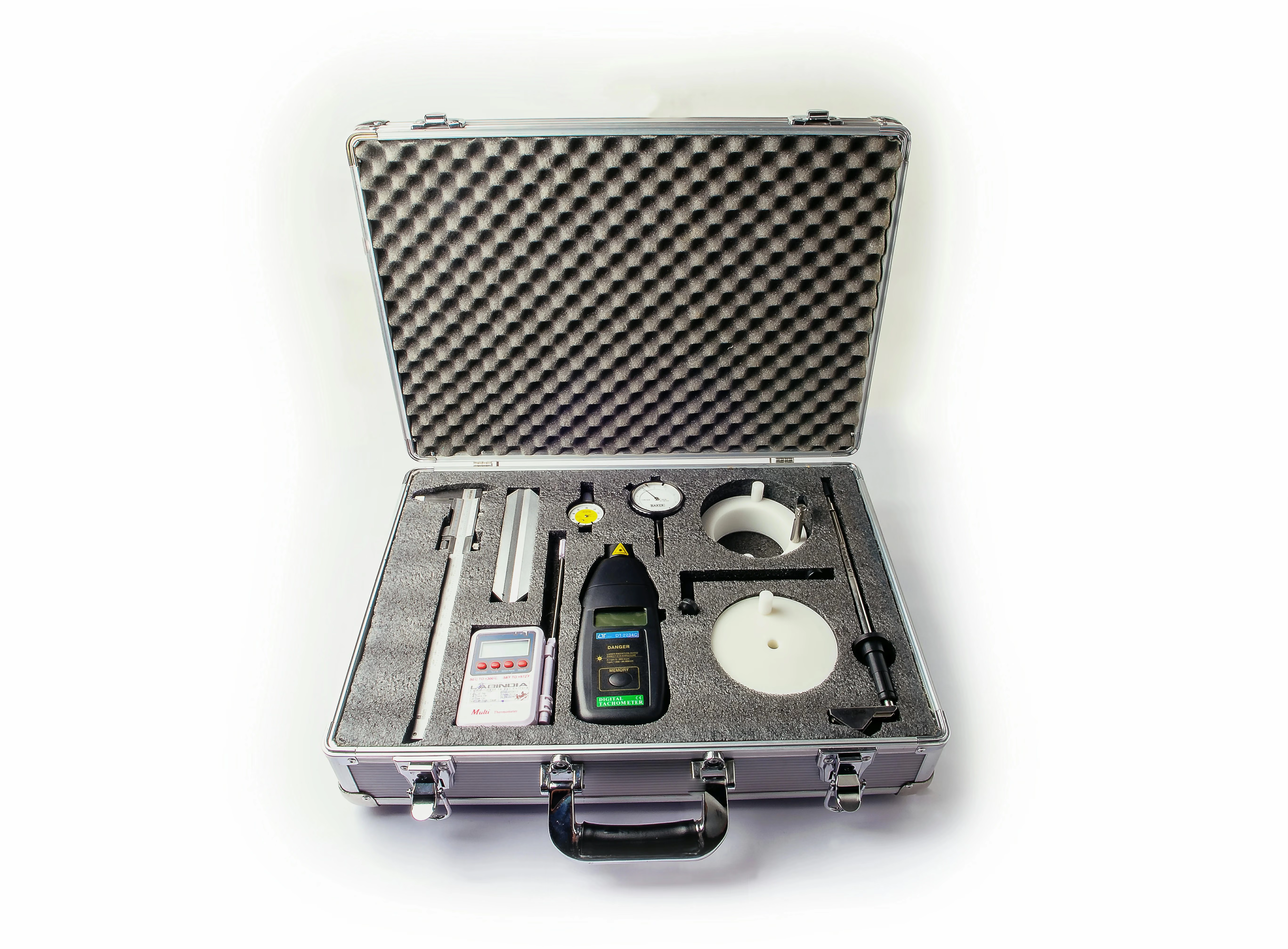
Dissolution Tester Mechanical Calibration Toolkit
Labindia provide mechanical qualifications that meet both USP and ASTM procedures.The dissolution toolkit provides a description of best practices associated with the mechanical calibration and performance verification test for the USP basket and paddle dissolution apparatuses and test assemblies. The best practices have been developed based on experience gained by the USP laboratory and with suggestions from the USP Expert Committee on Biopharmaceutics. While not a standard requiring rigid compliance, the dissolution toolkit is intended to provide information aiding the dissolution laboratory in the effort to obtain valid dissolution testing results.
Call On: +91 9136909578
Sales Support: sales.mfd@labindia.com
Service Support: servicecoordinator@labindia.com
More Types
Wobble Test Kit
Get Quote
Get Quote
X
Request Demo
Request Demo
X
Vibration Meter
Get Quote
Get Quote
X
Request Demo
Request Demo
X
Tachometer
Get Quote
Get Quote
X
Request Demo
Request Demo
X
Inside Divider
Get Quote
Get Quote
X
Request Demo
Request Demo
X
Digital Protractor
Get Quote
Get Quote
X
Request Demo
Request Demo
X
Variable Venier Depth Gauge
Get Quote
Get Quote
X
Request Demo
Request Demo
X
Tablet Dissolution Testers
Tablet Dissolution Tester DS 8000 (Basic) Tablet Dissolution Tester DS 14000 (Basic) Dissolution Apparatus DS 8000 with Peristaltic Pump Tablet Dissolution Tester DS 14000 with Peristaltic Pump Tablet Dissolution Tester DS 8000
+ with Syringe Pump Tablet Dissolution Tester DS 8000
+ with Piston Pump Tablet Dissolution Tester DS 14000
+ with Piston Pump Tablet Dissolution Tester DS 8000
+ with Piston Pump & Automatic Filter Changer Tablet Dissolution Tester DS 8000 (Basic) SMART Tablet Dissolution Tester DS 14000 (Basic) SMART Tablet Dissolution Tester DS 8000 SMART with Piston Pump Tablet Dissolution Tester DS 14000 SMART with Piston Pump Dissolution Accessories Dissolution Tester DS 14000
+ with Syringe Pump
